 Call Us Today At 1.866.845.3791
Call Us Today At 1.866.845.3791
RemedyRepack has one of the few modern, compliant facilities designed to avoid cross-contamination between penicillin and non-penicillin beta-lactam products. Isolated suites with independent packaging lines, HVAC air handling units, packaging equipment, utility systems, and dedicated staff are all possible with several facilities.
In addition, under the regulation Current Good Manufacturing Practice (cGMP), manufacturers must ensure that non-penicillin drugs do not contain detectable traces of penicillin compounds. The cGMP helps manufacturers maintain the highest safety standards by recalling products that pose health hazards to consumers. The Food and Drug Administration (FDA) has established an industrial guideline that insists on manufacture-separation protocols.
You can choose from a wide array of packaging solutions for these unique drug products, including HDPE bottles, unit-dose strip packs, and 30 day blister cards.


RemedyRepack only uses the highest integrity HDPE pharmaceutical bottles and closures. We understand that regulatory compliance, cGMPs, and validation are critical to the pharmaceutical industry. Therefore, we make every effort to source our packaging sustainably. In addition, using quality containers affords us the ability to offer maximum expiration allowed by regulation.
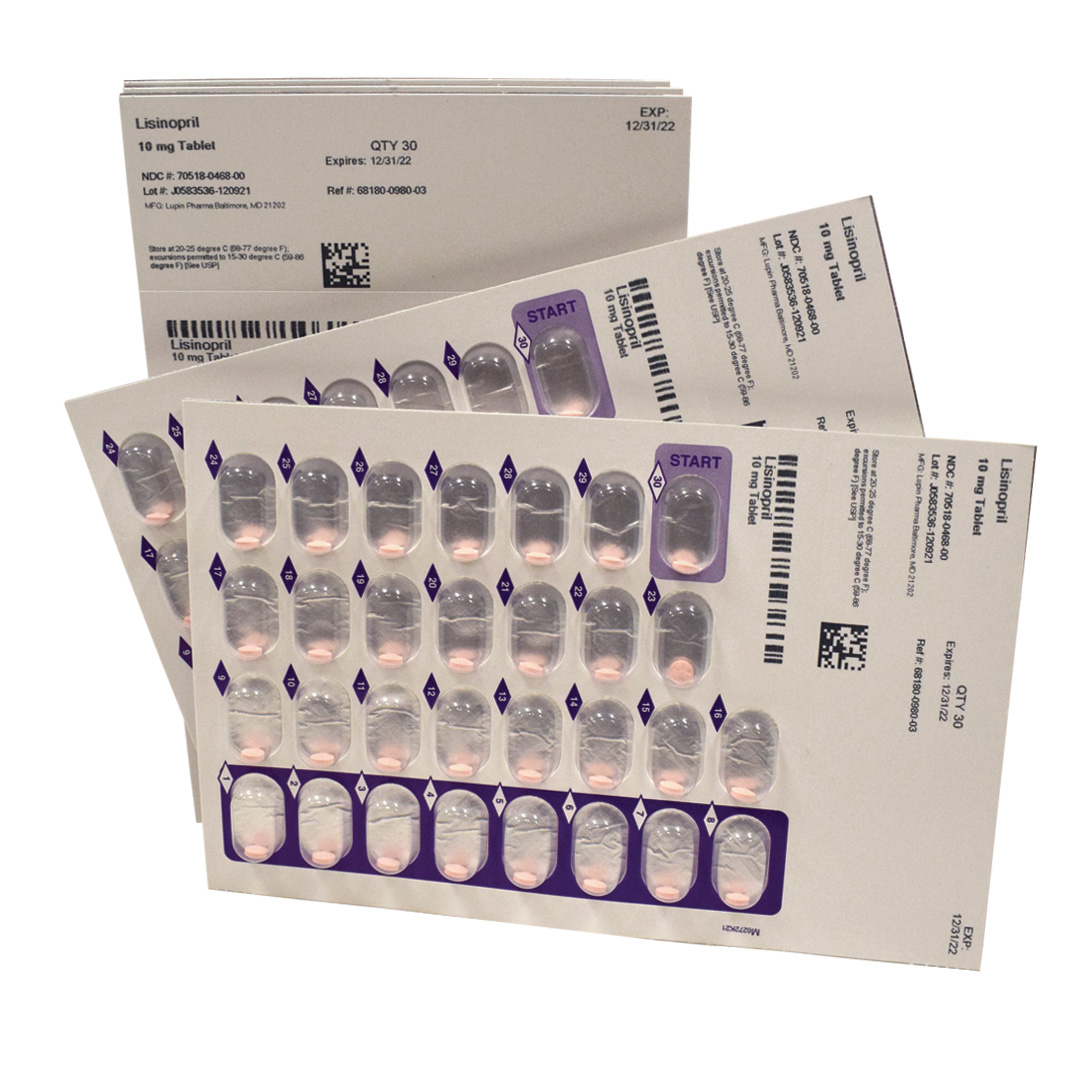
Long-term care, assisted living, and correctional facilities have long relied on RemedyRepack as one of the largest producers of prefilled blister cards for institutional pharmacies. Our blister cards are manufactured in the U.S. and meet all requirements for class A containers. Each cavity is uniquely barcoded and imprinted with all the required data. These inscriptions support the healthcare provider with medication management and, ultimately, medication-related patient outcomes. In addition, this easy-to-use medication dispensing method clearly shows if pills were taken or missed and indicates when refills are needed.
At RemedyRepack, we are committed to providing our customers with a flexible and cost-effective solution for high-demand and difficult-to-find unit-dose products. Outsourcing makes sense because it reduces direct expenses associated with capital equipment, repackaging supplies, and paying highly trained professionals to perform and manage non-core work. In addition, our clients can be confident that each unit dose package will be produced in adherence to industry guidelines for light, moisture, and sealing. All regulatoryly required medication information and barcodes are printed on quality thermal paper, which is smudge-free and fade-resistant. The reverse transparent side allows for clear visibility of the packaged medication. We are pleased to offer over 100 solid and liquid unit-dose products.